How to Calculate Formal Charge
Formula to Calculate the Formal Charge. CF group number of the atom - number of bonds it forms - number of unshared electrons If the atom has a CF with a value of 1 it is assigned a positive charge.

Calculating No3 Formal Charges Calculating Formal Charges For No3 Teaching Chemistry Chemistry Classroom Science Chemistry
The formula used to calculate formal charge.

. The formal charge on an atom in a molecule or ion is equal to the total number of valence electrons in the free atom minus the. Take the valence number of the atom and subtract the number of bonds and the number of non-bonding. The easiest way to calculate the formal charge of an atom is to subtract the number of dots and bonds around the atom from its group number where the groups are numbered as shown.
VE Valence Electrons. Formal charge H 1 valence e 0 nonbonding e 2 bonding e 2 0. According to a mathematical perspective the formal charge can be understood like this.
How to calculate formal charge using simple subtraction. Basically we can define formal charge as the hypothetical measure of charge assigned to an atom in a molecule assuming that electrons in all chemical bonds are shared equally between. Using the formula charge formula for each atom present we can calculate.
FC Formal Charge. The formal charges when added. The number of valence electrons equals to the elements group column in the periodic table.
Formal charges are important because they allow us to predict which Lewis structure is the mo. We can use this information to calculate the preferred Lewis structure of a molecule. The formal charge is derived from the number of valence electrons in a neutral atom.
Charges Calculate the formal charge on the sulfur highlighted in red in the molecule shown. Whereas if you have a. Formal charge exists because of deficiencies in the configuration of an atom that participates in the compound formation.
This chemistry video tutorial provides a basic introduction into how to calculate the formal charge of an atom or element in a lewis structure. FCO 6 ½ 6 2. FC VE 05BE NBE.
Calculation of formal charge is the subject of this. This way carbon has 4 oxygen has 6 and. To calculate formal charge of an atom use the equation below.
Formal charge valence electrons nonbonding electrons 12 bonding electrons B Bonding electrons total electrons shared in. Using the formula to calculate the formal charge on hydrogen we obtain. H3C S CH3 O Calculating some Formal Charges Sulfur is in Group IV.
The formal charge of an atom is calculated by subtracting the number of electrons assigned to that atom in the Lewis structure from the number of protons in the nucleus of that atom. Calculate the formal charge of the compound using the Lewis Dot structure in step 1 and the formula given. Formal charge Valence Electrons Sticks Dots.
Comment below with your questions and comments - dont forget to subscribe for more videos. A step-by-step description on how to calculate formal charges.

A Key Skill How To Calculate Formal Charge Education Help Science Lessons Organic Chemistry
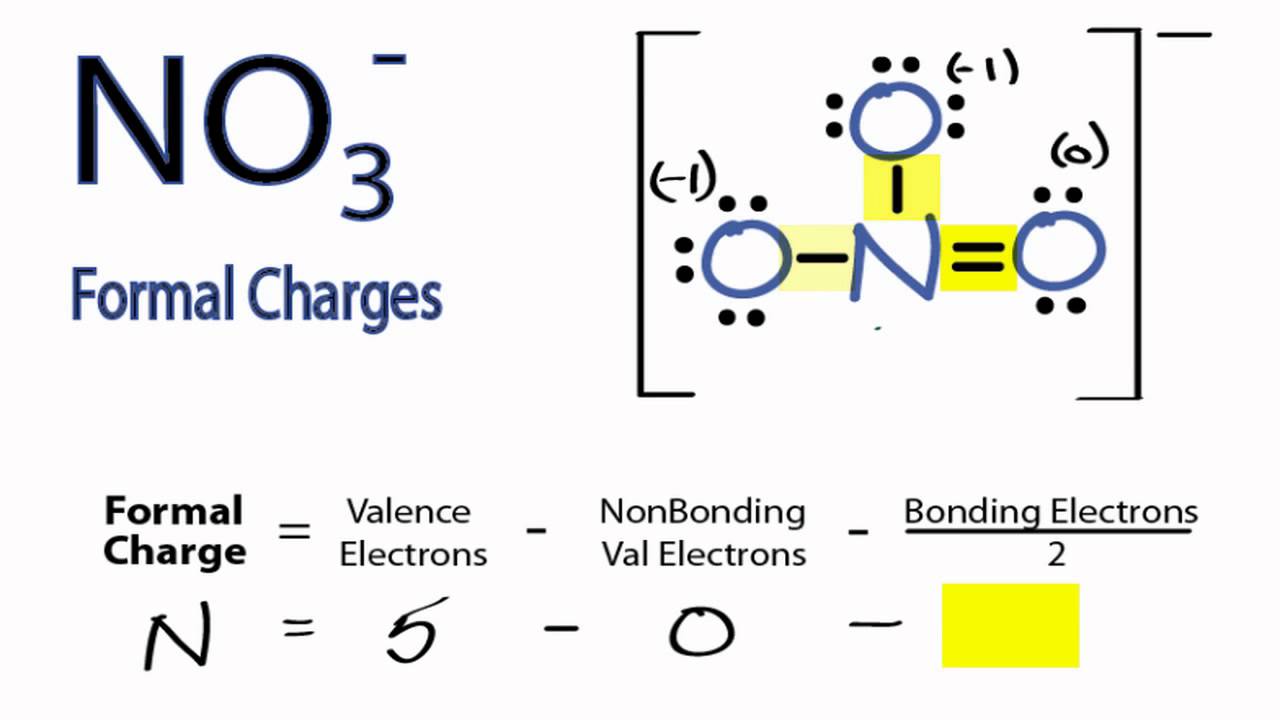
Calculating No3 Formal Charges Calculating Formal Charges For No3 Teaching Chemistry Chemistry Classroom Science Chemistry

Lewis Structure Formal Charge Calculation

Calculating No3 Formal Charges Calculating Formal Charges For No3 Teaching Chemistry Chemistry Classroom Science Chemistry
0 Response to "How to Calculate Formal Charge"
Post a Comment